Benzene – C6H6

Properties of Benzene
Melting Point: 5.5°C
Density: 876.50 kg/m³
The discovery of Benzene
Benzene was first isolated in 1825 by Michael Faraday from an oily film that deposited from the gas used for lighting. In 1833, it was the discovered again by Eilhard Mitscherlich who distilled it from gum benzoin. Benzene dissolve many organic and greasy substances so found a use in removing paint and stains from metal surfaces.
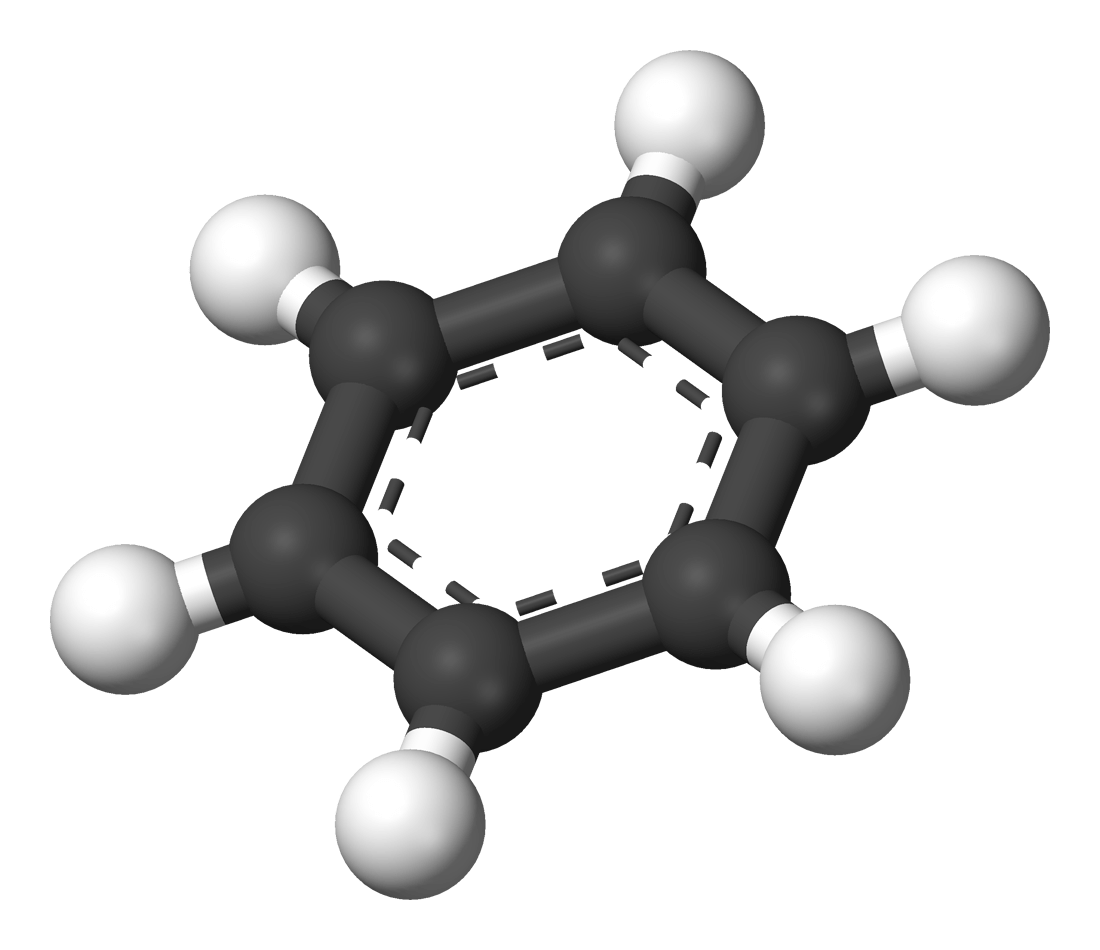
The Structure of Benzene
The first person to suggest the correct structure of Benzene was a man called Kekulé. He suggested that the carbons should be arranged in a hexagon with alternating double bonds and one hydrogen attached to each carbon.
Reactions of Benzene
Due to the double bonds you might expect benzene to react like an alkene which usually undergo elecrophilic additions, however benzene tends to undergo substitution. This is so that the benzene ring is still left in place when reacted as it would not be if an addition reaction were to take place across one of the double bonds.
Halogenation of Benzene
Benzene with react with either Chlorine or Bromine in an electrophilic substitution reaction but only when it is in the presence of a catalyst. This catalyst could either be aluminum chloride/bromide or iron
Electrophilic Substitution Mechanism
Stage one
Stage two