Born: October 25, 1875 in Weymouth, Massachusetts.
He recieved a PhD from Harvard University in 1899. After this he taught at Harvard and the Massachusetts Insitiute of Technology before he became the dean of the College of Chemistry at the University of California, Berkeley.
Lewis is notable for his idea of the “cubic atom”. With this he explained the eight groups in the periodic table and represented his idea that chemical bonds are formed by electron transfer to give each atom a complete outer shell. His ideas of chemical bonding continued to evolve and in 1916 he published his seminal article suggesting that a chemical bond is a pair of electrons shared by two atoms. This is known as the covalent bond.


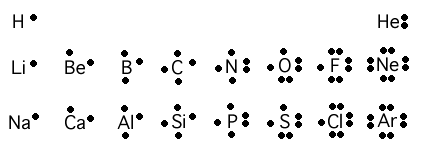
He also invented dot cross diagrams to show the movement of electrons between atoms. These are still used today, anyone doing GCSE chemistry will know about these!
Gilbert Lewis also made other contributions to the theories of colored substances, radiation, relativity, the separation of isotopes, heavy water, photochemistry, phosphorescence and fluorescence. During the First World War Lewis was a major in the US Army Chemical Warfare Service. He worked on defence systems against poisonous gases. During the period of 1922-1935 Lewis was nominated many times for the Nobel prize in chemistry. However on March 23rd 1946, while measuring the dielectric constant of hydrogen cyanide in his lab, Lewis died having not recieved his well deserved Nobel Prize.
